The global salt market size was valued at USD 14.16 billion in 2019, it was estimated to grow from USD 14.61 billion in 2023 to USD 19.4 billion by 2031.
Basically, salt has a huge consumption, and production, it’s an industry that makes billions of dollars every year.
It is also a necessity.
Not only is it essential for bodily functions like muscle contractions, but everybody needs it in their food and to preserve it, it’s also used in the manufacturing processes of soap, and industry chemicals like Sodium Hydroxide and Sodium Bicarbonate (Baking Soda), as well as a flux in metallurgy.
Essentially, salt is VERY vital to us.
Salt can be acquired through seawater, deep-shaft mining, and salt brines; the impure salt harvested through mining (rock salt/halites), and seawater evaporation (sea salt) can contain algae, sand, organic matter, etc. and is thus, often purified to common salt depending on manufacturing practices (grain size, purity, sodium content).
There are also artisans that practice the production of fleur de sel, salt acquired through old-fashioned techniques to harvest a luxury product, often sold for $420 per kilogram in the USA.
A major source of common salt is seawater, which has a salinity of approximately 3.5%. Meaning that there are about 35 g (1.2 oz) of dissolved salts, predominantly sodium (Na+ ) and chloride (Cl− ) ions, per kilogram (2.2 lbs) of water. As common salt is a mineral composed primarily of Sodium Chloride, NaCl, and approximately 70% of Earth’s surface is seawater – there’s a lot of salt to go around.
The principle of salt production is one that links up basic ideas of kinetic theory, and molecular thematics – involving evaporating the water from seabrines or areas of concentrated seawater.
The process goes something like this:
- Saltwater is collected from either seawater or saline lakes that contain dissolved salts (primarily NaCl).
- The water undergoes initial purification to remove large debris / physical impurities like sand, shells, organic matter. This can be done via filtration or settling processes.
Filtration relies on the separation of particles based on either size or solubility. In this context, done by passing seawater through filters to remove larger particles ie. sand, shells that may be floating in the water.
Settling relies on the fact that denser particles will sink to the bottom of the container – when the particles have not dissolved into the solution. This is also called sedimentation or gravitational settling. This is done by allowing the saltwater to sit still for a while, letting the heavier particles / impurities settle.
- This purified seawater is then transferred to salt evaporation ponds, located in areas with high temperatures, ample sunlight and low rates of rainfall (usually near the source of seawater) to maximize the evaporation that takes place.

Evaporation occurs when particles possess enough energy to escape from the surface of the liquid. In this case, the optimal conditions for the water molecules to gain enough energy from the rapid heating (high temperature, energy from the sun) speed up the evaporation – decreasing the water content in the solution quickly.
As the water content in the salt solution decreases, it becomes a salt concentrated solution.
- Crystallization occurs – salt crystals are formed.
As the concentration of salt in the water grows, a saturated / concentrated solution is made. When the solubility of water has been reached, the maximum amount of salt possible has been dissolved in the water available.
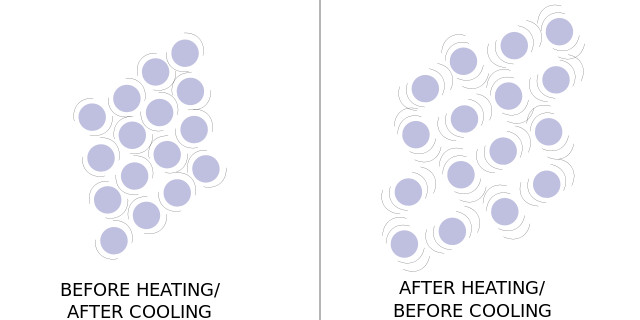
At a molecular level, the salt particles bridge the gaps between the water molecules – and due to the high temperature and energy transfer, the spaces between the water molecules increase as they tend to move faster and drift further away.
This allows the solubility of the water to increase, as there is more space between for the salt to be contained. This means that the utmost MAXIMUM amount of salt is contained in the water, and that’s it, no more.
However, as night comes, for example – contraction occurs. Due to the solution cooling down, the water molecules come closer together as their vibrations slow down, and as the solution contracts, the salt ions are more likely to come together and form lattice structures – in the form of halites / salt crystals. This can also be caused due to further evaporation of water, pushing the salt ions together to form a structure.
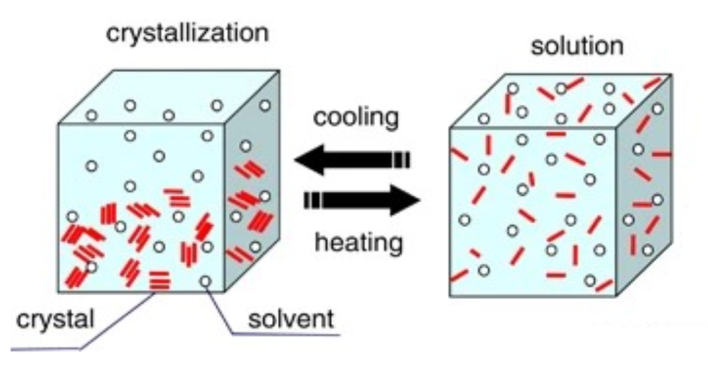
These structures contribute to the growth of bigger crystals and them growing more structured and large. Eventually, the crystals can no longer grow, as the content and chemical composition of the solution has undergone change to a point where it won’t matter whether expansion or contraction occurs.

- These crystals are then harvested, dried, and packaged depending on manufacturing needs. Some industry plants may require further purification or breakdown from seasalt to common salt, but that all comes down to commercial preference, and safety protocols.
This is the primary process that supports a billion dollar industry, boiling down to basic physics and chemistry. It’s not rocket science.
So, if you think about it, the next time you go to the beach you could just fill a bottle of seawater, and make your own salt.
By Sharvina Srivastava



Leave a comment